The federal multidistrict litigation against Cook Medical took another positive step recently. Cook Medical and plaintiffs have agreed to bellwether cases that will be prepared for early trial dates.
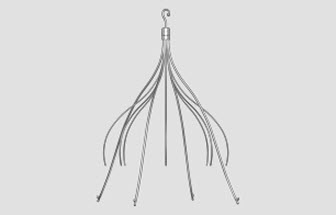
All federal product liability and personal injury lawsuits over complications with Cook Celect and Cook Gunther Tulip IVC filters have been centralized before U.S. District Judge Richard Young in the Southern District of Indiana, as part of an MDL, or multidistrict litigation.
There are currently 400 lawsuits filed by patients with serious injuries, according to the latest court data as of May 16.
The small, umbrella like devices are implanted for prevention of pulmonary embolism and they perforate the vena cava, migrated out of position or fracture, sending fragments or metal shards into the heart or lungs.
Cook IVC Filter September 2016 Bellwether Trials
Judge Young announced a “bellwether” process, in which a group of cases will be prepared for early trial dates, expected to begin in September, 2016.
The bellwether process is common in complex nationwide pharmaceutical and medical device lawsuits and is developed to push the parties to IVC blood clot filter settlement negotiations.
What are IVC Filters?
The IVC filters are implanted in the inferior vena cava (IVC) of patients at risk of suffering a pulmonary embolism, and are designed to be removed after the risk of a blood clot has passed.
Plaintiffs claim that the device has design defects that make them subject to failure, often requiring risky surgery to remove the IVC filter or leave patients with the device lodged permanently inside their body, where it may cause further injury or death if it migrates out of position.
Bard IVC Filter Bellwether Trials
A bellwether program has been set up in the federal IVC filter litigation against C.R. Bard, which manufactured similarly designed blood clot filters and have also been linked to similar side effect problems.
There are currently over 500 lawsuits filed by patients with serious injuries, where the devices were implanted for prevention of pulmonary embolism and perforated the vena cava, migrated out of position or fractured, sending fragments or metal shards into the heart or lungs.
Bard Recovery filter and Bard G2 filter lawsuits are pending in a MDL, which is centralized before U.S. District Judge David Campbell in the District of Arizona. Bellwether selections in the Bard IVC filter MDL are not likely until 2017.




