Bard is under pressure with thousands of product liability lawsuits, including cases involving vaginal mesh implants and IVC filters. There are a growing number of lawsuits have been filed against the medical device company, including more than 8,000 Bard Avaulta vaginal mesh lawsuits and several dozen lawsuits over Bard IVC (inferior vena cava) filters.

Avaulta TransVaginal Mesh Lawsuits
Bard’s Avaulta transvaginal mesh have been used for surgical repair of pelvic organ prolapse (POP) and female stress urinary incontinence (SUI). Avaulta mesh has been recalled after reports indicated that women were suffering severe and catastrophic injuries from mesh erosion through the vagina.
The injured women are claiming that Bard designed and sold a defective and dangerous product. According to experts, Bard used plastic in the vaginal mesh that a supplier informed them was not fit for permanent implant in humans.
Next week, a Bard Avaulta trial is scheduled, with the first in a series of four “bellwether” cases that have been set for early trial dates. The successful outcome for the plaintiffs may influence negotiations with Bard to settle Avaulta vaginal mesh cases.
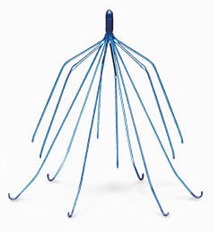
Bard IVC Filter Side Effects
Inferior vena cava (IVC) filters, are devices implanted in veins to prevent blood clots before they travel into the lungs, causing pulmonary embolism. According to the complaints, the struts of the Bard IVC filters can fracture or break off, causing serious injury when they migrate to other areas of the body.
Many Bard IVC filter lawsuits have been filed in state and federal courts and the FDA issued an alert in August 2010 about side effects associated with such ivc filters.
The FDA reported that it had received hundreds of adverse event reports involving the Bard G2 and Bard Recovery filters. These filters are designed to be left in place or removed by the doctor after the danger of a blood clot has passed. The FDA urged doctors to remove the filters, to reduce the risk of the IVC filter breaking free, perforating the vena cava or causing other internal injuries.
Bard introduced the Recovery IVC filter in 2002 and introduced the G2 filter as a second generation version. The Bard G2 was marketed as having “enhanced fracture resistance”, “improved centering” and “increased migration resistance.” The new version is also plagued by side effects.
Plaintiffs allege that Bard knew or should have known about the high rate of fracture, migration and perforation with their vena cava filters, and failed to adequately warn consumers or the doctors.




